Recently, Jiaxing Market Supervision Administration conducted a comprehensive sampling of the process water of Haiyan Kangyuan Medical Instrument Co., LTD., and announced that the process water of Kangyuan Medical is fully in line with the purified water requirements of the 2020 edition of Chinese Pharmacopoeia, which validates the excellent control ability of Kangyuan Medical in product quality and patient safety.
The sampling inspection was organized by Jiaxing Market Supervision Administration and commissioned by Jiaxing Food, Drug and Product Quality Inspection and Testing Institute. In accordance with relevant national standards and industry norms, the inspection and testing Institute has conducted a comprehensive and professional test on the process water used by Kangyuan Medical to produce various medical devices, including water pH, nitrate, conductivity, heavy metals, microbial limits and many other aspects. After several rounds of rigorous testing, the results show that the process water of Kangyuan Medical fully meets the purified water requirements of the 2020 edition of Chinese Pharmacopoeia, which fully guarantees the quality safety and reliability of Kangyuan medical device products.
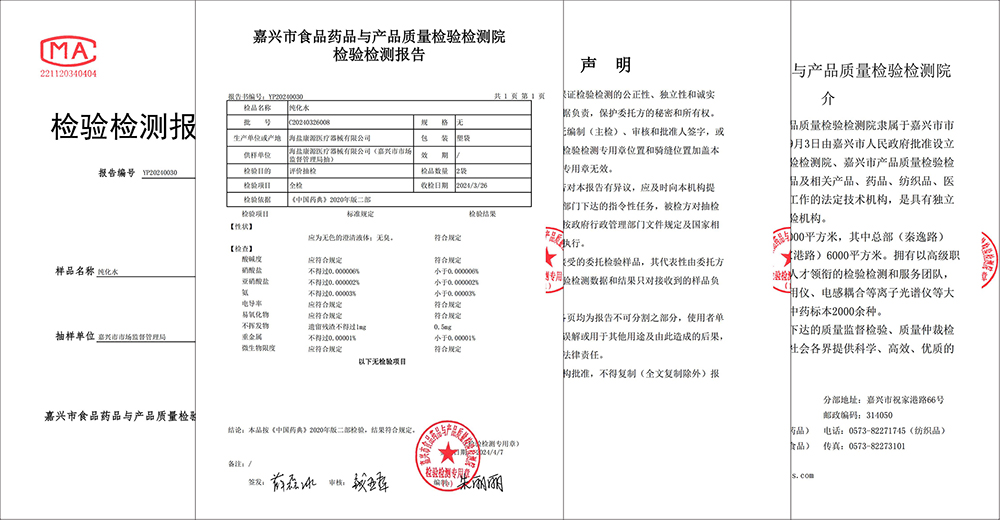
Kangyuan Medical has always put product quality and patient safety in the first place, and attaches particular importance to the quality control of process water. The company has introduced advanced water production equipment and monitoring technology, and established a sound process water management system and operating procedures to ensure that every drop of water meets the national standards. The passage of the sampling inspection is not only an affirmation of the quality of Kangyuan medical process water, but also an recognition of the overall quality management system of Kangyuan Medical.
In the future, Kangyuan Medical will uphold its profound industry accumulation and the spirit of continuous innovation, continue to play a leading role in the medical industry, to ensure that the majority of patients with better quality and safe medical consumables, to make greater contributions to the cause of human health.
Post time: May-29-2024

 中文
中文