Manufacturer for Foley Catheter Urinary - Silicone Foley Catheter with Temperature Probe – Kangyuan
Manufacturer for Foley Catheter Urinary - Silicone Foley Catheter with Temperature Probe – Kangyuan Detail:
![]()
Packing: 10 pcs/box, 200 pcs/carton
Carton size: 52x34x25 cm
It is used for routine clinical urethral catheterization or urethral drainage for continuous monitoring of patients’ bladder temperature with a monitor.
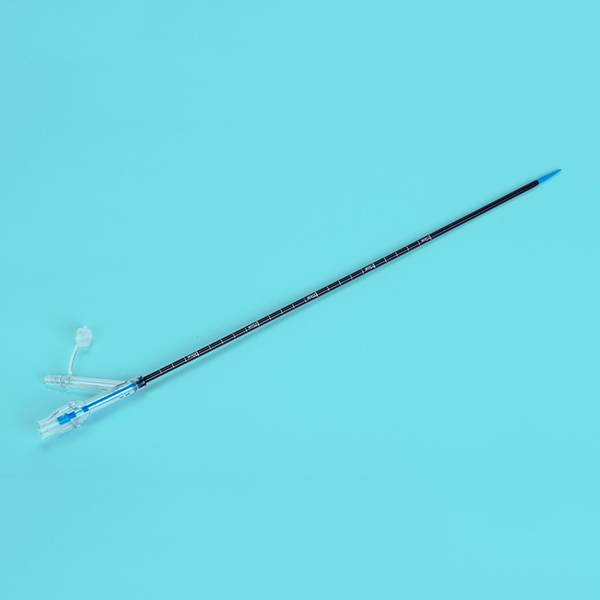
This product is composed of urethral drainage catheter and temperature probe. Urethral drainage catheter consists of catheter body, balloon (water sac), guide head (tip), drainage lumen interface, filling lumen interface, temperature measuring lumen interface, flushing lumen interface (or no), flushing lumen plug (or no) and air valve. Temperature probe consists of temperature probe (thermal chip), plug interface and guide wire composition. Catheter for children(8Fr, 10Fr) can include a guide wire(optional). The catheter body, guide head (tip), balloon (water sac) and each lumen interface are made of silicone; the air valve is made of polycarbonate, ABS plastic and polypropylene; the flushing plug is made of PVC and polypropylene; the guide wire is made of PET plastic and temperature probe is made of PVC, fiber and metal material.
This product is equipped with a thermistor which senses the core temperature of the bladder. The measuring range is 25℃ to 45℃, and the accuracy is ±0.2℃. 150 seconds balance time should be used before measurement. The strength, connector separation force, balloon reliability, bending resistance and flow rate of this product shall meet the requirements of ISO20696:2018 standard; meet the electromagnetic compatibility requirements of IEC60601-1-2:2004; meet the electrical safety requirements of IEC60601-1:2015. This product is sterile and sterilized by ethylene oxide. The residual amount of ethylene oxide should less than 10 μg/g.
|
Nominal Specification |
Balloon Volume (ml) |
Identification color code |
||
|
Articles |
French Specification(Fr/Ch) |
Nominal external diameter of catheter pipe(mm) |
||
|
second lumen, third lumen |
8 |
2.7 |
3, 5, 3-5 |
pale blue |
|
10 |
3.3 |
3, 5, 10, 3-5, 5-10 |
black |
|
|
12 |
4.0 |
5, 10, 15, 5-10, 5-15 |
white |
|
|
14 |
4.7 |
5, 10, 15, 20, 30, 5-10, 5-15, 10-20, 10-30, 15-20, 15-30, 20-30 |
green |
|
|
16 |
5.3 |
orange |
||
|
Second lumen, third lumen, forth lumen |
18 |
6.0 |
5, 10, 15, 20, 30, 50, 5-10, 5-15, 10-20, 10-30, 15-20, 15-30, 20-30, 30-50 |
red |
|
20 |
6.7 |
yellow |
||
|
22 |
7.3 |
purple |
||
|
24 |
8.0 |
blue |
||
|
26 |
8.7 |
pink |
||
1. Lubrication: the catheter should be lubricated with medical lubricant before insertion.
2. Insertion: insert the lubricated catheter into the urethra to the bladder carefully (urine is discharged at this time), then insert 3-6cm and make the balloon completely enter the bladder.
3. Inflating water: Using a syringe without needle, inflate balloon with sterile distilled water or 10% glycerin aqueous solution is supplied. Recommended volume to use is marked on funnel of catheter.
4. Temperature measuring: if necessary, connect the external end interface of the temperature probe with the socket of the monitor. Patients’ temperature can be monitored in actual time through the data displayed by the monitor.
5. Remove: When removing the catheter, firstly separate the temperature line interface from the monitor, insert an empty syringe without needle into the valve, and suction sterile water in the balloon. When the volume of water in the syringe is close to that of the injection, the catheter can be pulled out slowly, or the tube body can be cut off to remove the catheter after rapid drainage.
6. Indwelling: Indwelling time depends on clinical needs and nursing requirements, but the maximum indwelling time shall not exceed 28 days.
1. Acute urethritis.
2. Acute prostatitis.
3. Failure of intubation for pelvic fracture and urethral injury.
4. Patients considered unsuitable by clinicians.
1. When lubricating the catheter, do not use lubricant containing oil substrate. For example, using paraffin oil as a lubricant will causes balloon rupture.
2. Different sizes of catheters should be selected according to age before use.
3. Before use, check whether the catheter is intact, whether the balloon is leaking or not, and whether the suction is not unobstructed. After connecting the temperature probe plug with the monitor, whether the data displayed is abnormal or not.
4. Please check before use. If any single (packed) product is found to have the following conditions, it is strictly prohibited to use:
A) beyond the expiration date of sterilization;
B) the single package of the product is damaged or has foreign matters.
5. The medical staff should take gentle actions during intubation or extubation, and take good care of the patient at any time during the indwelling catheterization to prevent accidents.
Special note: when the urine tube indwelling after 14 days, in order to avoid the tube may slip out due to the physical volatilization of sterile water in the balloon, the medical staff can inject sterile water into the balloon in one time. The operation method is as follows: keep the urine tube in retained state, draw the sterile water out of the balloon with a syringe, then inject sterile water into the balloon according to the nominal capacity.
6. Insert the guide wire into the drainage lumen of the catheter for children as an auxiliary intubation. Please draw out the guide wire after intubation.
7. This product is sterilized by ethylene oxide and has a valid period of three years from the date of production.
8. This product is disposable for clinical use, operated by medical personnel, and destroyed after use.
9. Without verification, it shall be avoided to use in the scanning process of the nuclear magnetic resonance system to prevent potential interference that may lead to inaccurate temperature measuring performance.
10. The leakage current of the patient shall be measured between the ground and thermistor at 110% of the highest rated network supply voltage value.
1. Portable multi-parameter monitor (model mec-1000) is recommended for this product;
2. i/p: 100-240V-,50/60Hz, 1.1-0.5A.
3. This product is compatible with YSI400 temperature monitoring system.
1.This product and the connected monitor equipment shall take special precautions regarding electromagnetic compatibility (EMC) and shall be installed and used in accordance with the electromagnetic compatibility information specified in this instruction.
The product must use the following cables to meet the requirements of electromagnetic emission and anti-interference:
|
Cable name |
length |
|
Power line(16A) |
<3m |
2. The use of accessories, sensors and cables outside the specified range may increase the electromagnetic emission of the equipment and/or reduce the electromagnetic immunity of the equipment.
3. This product and the connected monitoring device cannot be used close to or stacked with other devices. If necessary, close observation and verification shall be conducted to ensure its normal operation in the configuration used.
4. When the input signal amplitude is lower than the minimum amplitude specified in the technical specifications, the measurement may be inaccurate.
5. Even if other equipment complies with the launching requirements of CISPR, it may cause interference to this equipment.
6. Portable and mobile communication devices will affect the performance of the device.
7. Other devices containing RF emission may affect the device (e.g. cell phone, PDA, computer with wireless function).
[Registered person]
Manufacturer: HAIYAN KANGYUAN MEDICAL INSTRUMENT CO., LTD
Product detail pictures:
Related Product Guide:
Gaining purchaser gratification is our company's aim eternally. We're going to make great initiatives to create new and top-quality products, satisfy your exclusive prerequisites and supply you with pre-sale, on-sale and after-sale solutions for Manufacturer for Foley Catheter Urinary - Silicone Foley Catheter with Temperature Probe – Kangyuan, The product will supply to all over the world, such as: Senegal, Kenya, Sweden, When It produced, it making use of the world's major method for reliable operation, a low failure price, it appropriate for Jeddah shoppers choice. Our enterprise. s situated inside the national civilized cities, the website traffic is very hassle-free, unique geographical and financial circumstances. We pursue a "people-oriented, meticulous manufacturing, brainstorm, make brilliant" company philosophy. Strict good quality management, fantastic service, affordable cost in Jeddah is our stand around the premise of competitors. If needed, welcome to make contact with us by our web page or phone consultation, we'll be delighted to serve you.
The company's products can meet our diverse needs, and the price is cheap, the most important is that the quality is also very nice.

 中文
中文