Disposable Breathing Filter
Packing: 200pcs/carton
Carton size: 52x42x35 cm
This product is associated with anesthesia breathing equipment and lung function instrument, used to filter particles in the air above 0.5μm.
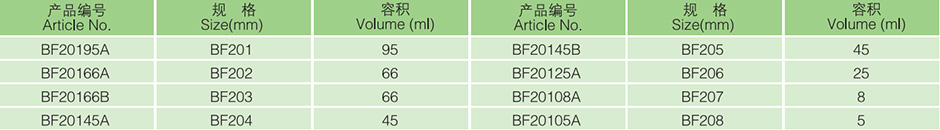
|
specification |
1# |
2# |
3# |
4# |
5# |
6# |
7# |
8# |
|
volume (ml) |
95ml |
66ml |
66ml |
45ml |
45ml |
25ml |
8ml |
5ml |
|
upper cover form |
Straight type |
Straight type |
Elbow type |
Straight type |
Elbow type |
/Straight type |
Straight type |
Straight type |
Disposable breathing filter (commonly known as: artificial nose), it consists of the upper cover, the lower cover, filter membrane, protective cap composition. Among them: the upper cover of the respiratory filter, the lower cover is made of ABS material or polypropylene material, the filter membrane is made of polypropylene composite material. The filter rate of the product is not less than 90%. 0.5μm particles in the air.
1. Open the package, take out the product, according to the patient to select the appropriate specifications of the model of the respiratory filter.
2. According to the patient's anesthesia or breathing routine operation mode, the two port connector of the breathing filter is connected with the breathing pipe or instrument.
3. Check the pipeline interface is strong, should prevent the accidental fall off in use, can be used when necessary tape fixed.
4. The general use of breathing filter time is not more than 48 hours, it is best to replace every 24 hours once, not repeated use.
Excessive secretion of patients and patients with severe lung wet.
1. Before use should be based on age, weight of different choice of the correct specifications and testing product quality.
2. Please check before use, such as found in single (packaging) products have the following conditions, is strictly prohibited:
a) the effective period of sterilization failure;
b) the product is damaged or a single piece of foreign matter.
3. This product for clinical use, operation and use by the medical staff, after the destruction.
4. In use process, should pay attention to monitoring respiratory filter smoothness and no leakage, such as found in the patient's airway secretions (such as a large number of sputum), should be used to temporarily stop breathing filter; such as the discovery of respiratory filters are sputum pollution or blockage, should be the timely replacement of breathing filters; such as breathing filter joint release the leak occurs, should be immediately dealt with.
5. This product is sterile, sterilized by ethylene oxide.
[Storage]
Products should be stored in relative humidity of not more than 80%, no corrosive gas and good ventilation clean room.
[Date of manufacture] See inner packing label
[Expiry date] See inner packing label
[Registered person]
Manufacturer: HAIYAN KANGYUAN MEDICAL INSTRUMENT CO., LTD

 中文
中文