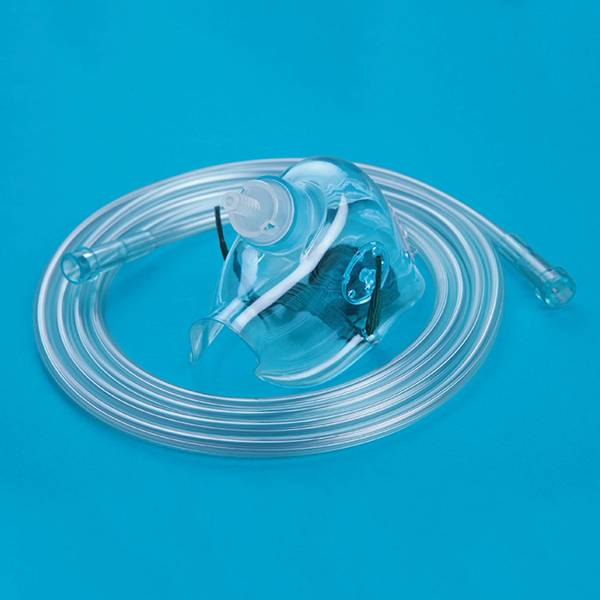
Anesthesia Breathing Circuits
Packing: 40 pcs/carton
Carton size: 75x64x58 cm
The product should be used together with the anesthesia machine, ventilator, tidal device and nebulizer for clinic patients to establish a respiratory connection channel.
1. Single pipe type(BCD101, BCD102, BCD201, BCD202)
2. Double pipes type(BCS101, BCS102, BCS201, BCS202)
Remark: depending on the chosen configuration, the manufacturer could increase codes that are edited by the manufacturer at the end of model specification.
1. Pipe ( soft pipe) OD: 18mm, 22mm, 25mm, 28mm;
2. Pipe (soft pipe) length, rated flow, the leakage rate is the mark on the packing bag.
Remark: customize products’dimension and parameter according to the order contracts' regulation.
The product is composed of the basic configuration components and the chosen configuration components. Basic configuration consists of a corrugated hose and various joints. Including: the corrugated hose contains single pipeline type telescopic and retractable and the dual pipeline type telescopic and retractable; joints comprise a joint 22mm/15mm, Y type joint, right angle or straight shaped adapter; Chosen configuration includes respiratory filter, a face mask, breathing bag subassembly. The corrugated hose of the product is made of PE, medical PVC material and the joint is made of PC and PP materials. Products are aseptic. If sterilized by ethylene oxide, ethylene oxide residue of the factory should be less than 10 g/g.
1. Open the packing and take out the product. According to the type and size of the configuration, check whether the product lacks accessories;
2. According to the clinical need, select the appropriate model and configuration; according to the patient's anesthesia or breathing routine operation mode, connecting the respiratory pipe components is ok.
Pneumothorax and mediastinal emphysema without drainage, pulmonary bulla, hemoptysis, acute myocardial infarction, bleeding shock not supplement the blood volume before, the use of mechanical ventilation is prohibited.
1. Before using, choosing correct specifications and testing product’s quality according to different age and weight.
2. Before using, PLS check. If single (packing) product has following conditions, it is prohibited to be used:
a. The valid period of sterilization is ineffective.
b. Single product’s packaging is damaged or has foreign matter.
3. The product is disposable for the clinical use. It is operated by medical personnel and will be destroyed after use.
4. In the use’s process, should pay attention to monitor the use’s matter of the breathing circuit. If the breathing circuit leaks and the joint looses, the product should be stoped to be used and medical personnel should deal with it.
5. The product is sterilized by ethylene oxide and the valid period of the sterilization is 2 years
6. If he packaging is damaged. The product is prohibited to be used.
[Storage]
Products should be stored in relative humidity of not more than 80%, no corrosive gas and good ventilation clean room.
[Date of manufacture] See inner packing label
[Expiry date] See inner packing label
[Registered person]
Manufacturer: HAIYAN KANGYUAN MEDICAL INSTRUMENT CO., LTD

 中文
中文